Some Important Compounds of Elements of S-block
Some Important Compounds of Elements of S-block: Overview
This topic covers concepts such as compounds of alkali metals, sodium carbonate, Solvay's process, physical properties of sodium carbonate, and crystalline decahydrate form of sodium carbonate.
Important Questions on Some Important Compounds of Elements of S-block
When is oxidised, the product is
When a substance A reacts with water it produces a combustible gas B and a solution of substance C in water. When another substance D reacts with this solution of C, it also produces the same gas B on warming but D can produce gas B on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A, B, C and D respectively are
The substance not likely to contain CaCO3 is
The products of the reaction between aqueous solutions of and are
In the given reaction cycle
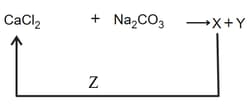
and respectively are
Number of water molecules in washing soda and soda ash respectively are :
Water of crystallization in soda ash and washing soda is respectively.
Write the preparation and properties of .
In which of the following reaction, acts neither as an oxidizer nor as a reducer
can be transported in the container made up of:
When we find n factor of , why did we do it with respect to oxygen?
is reduced to by using
A white salt reacts with an aqueous solution of to form a white precipitate. The salt can be:
During the Solvay process, _____ is one of the products formed when calcium hydroxide is reacted with ammonium chloride. A white solid is formed and disposed in oceans.
Why can't potassium carbonate be made by Solvay process?
What is washing soda? State two properties of washing soda.
A salt is obtained using common salt as a raw material. It is used in glass, soap and paper industries and also used in the manufacture of sodium compounds such as borax. Identify the salt.
What is the role of caustic soda in the industrial cleaning process?
What is sodium hydroxide?
How is sodium hydroxide prepared commercially.
